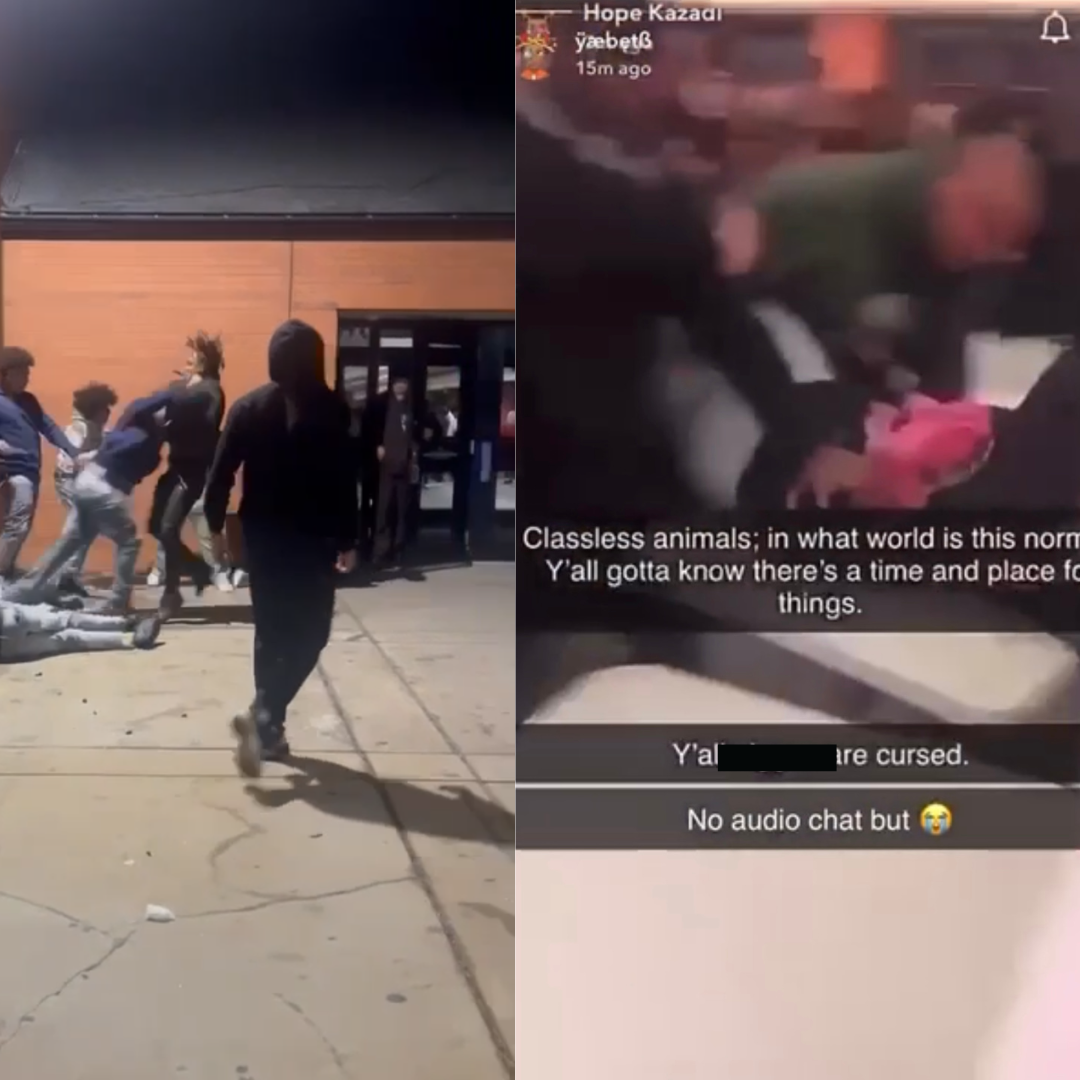
Handful of Flames
Mr. Behrman’s chemistry class experiments chemical reaction
January 15, 2016
L
Chemistry teacher Jonathan Behrman spent his 1R class teaching students to create chemical reactions.
In class students performed balancing equations, chemical reactions, and identifying the type of reaction that occurred.
Students took copper string and placed it in a test tube and poured silver nitrate into it, then covered the top to reduce the amount of oxygen in the mixture, making the reaction more vivid.
“The purpose is to expose them to different types of chemicals and their reactions with each other first hand,” Behrman said.
“The students will be using some very dangerous and hazardous chemicals, Alkali Metals, Natural gas, Magnesium Sodium hydroxide, Manganese Dioxide which are all pretty nasty and corrosive to human tissue,” Behrman said.
A chemical reaction is the interconversion of chemical substances. Usually chemical reactions show the motion of electrons in the forming and breaking of chemical bonds. Different chemical reactions are used to synthesize different desired products.
“I’ve done this for about 10 years now and you can expect to see it next year as well,” Behrman said.